Order YCANTH® Now
Permanent YCANTH J-code (J7354)
Permanent YCANTH J-code (J7354)
Buy & Bill
FFF Enterprises, Inc
Y-Access can assist with patient benefit investigations and automated copay enrollment for eligible patients.
1-855-YCANTHS (1-855-922-6847)Your practice will receive a welcome call within 1 business day.
Y-AccessSupport.com
To confirm a patient's insurance coverage

To enroll a patient in the Copay Assistance Program
Learn about the integrated benefits of the Y-Access Support Solutions Training Portal.
Access, billing, and coding support

Prescribing Information

FDA Approval Letter

Letter of Medical Necessity

Pooled Phase 3 Reprint
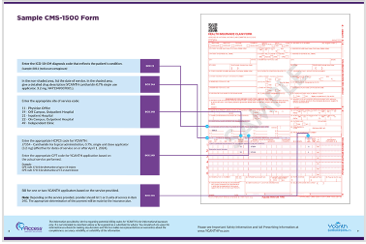
CMS 1500 Sample

CMS 1450 Sample

Billing and Coding Guide
Copay savings for commercially insured patients*
With the YCANTH Copay Assistance Program, commercially insured patients can pay just $25 per treatment visit, up to two applicators. Eligibility requirements apply.
Enroll your patients
Per treatment visit, up to two applicators:
Remind patients that flexible spending accounts (FSA) or health savings accounts (HSA) can help cover copay expenses. Healthcare expense accounts can help offset qualified out-of-pocket expenses. Encourage patients to check with their program provider. Additional terms and conditions may apply.
TRICARE® is a registered trademark of the Department of Defense (DOD), DHA.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: None.
WARNINGS AND PRECAUTIONS:
INDICATION
YCANTH (cantharidin) topical solution, 0.7% is indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: None.
WARNINGS AND PRECAUTIONS:
ADVERSE REACTIONS:
The most common (incidence ≥1%) reactions are the following local skin reactions at the application site: vesiculation, pain, pruritus, scabbing, erythema, discoloration, application site dryness, edema, and erosion. Local skin reactions at the application site were observed in 97% of subjects treated with YCANTH during clinical trials. These local skin reactions are expected and related to the anticipated blistering response of the skin to cantharidin.
DRUG INTERACTIONS:
No studies evaluating the drug interaction potential of cantharidin have been conducted.
USE IN SPECIFIC POPULATIONS:
Pregnancy: There are no available data with use of YCANTH in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Given that systemic exposure to cantharidin following topical administration is low, maternal use is not expected to result in fetal exposure to the drug.
Lactation: Avoid application of YCANTH topical solution to areas with increased risk for potential ingestion by or ocular exposure to the breastfeeding child.
OVERDOSAGE:
Oral ingestion of cantharidin has resulted in renal failure, blistering and severe damage to the gastrointestinal tract, coagulopathy, seizures, and flaccid paralysis.
Please see full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Verrica Pharmaceuticals Inc. at 1-877-VERRICA (1-877-837-7422), or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Local skin reactions are expected and should be reported if they are severe.
DHA=Defense Health Agency; DOD=Department of Defense; NDC=National Drug Code; VA=Veterans Affairs..